Vancomycin Risk Assessment Tool
Patient Risk Assessment
Risk Assessment Results
Nephrotoxicity Risk
Ototoxicity Risk
Recommendations:
Vancomycin saves lives. It’s one of the few antibiotics that can stop MRSA when nothing else works. But every time you give it, you’re walking a tightrope between saving a patient and damaging them. Two serious side effects loom large: nephrotoxicity and ototoxicity. One hurts the kidneys. The other steals hearing. Both can be permanent. And they don’t always show up the way you expect.
Why Vancomycin Is Still Necessary
Vancomycin has been around since the 1950s, but it’s not outdated. In 2025, it’s still the go-to for severe Gram-positive infections - especially MRSA, endocarditis, and hospital-acquired pneumonia. The Infectious Diseases Society of America says 89% of clinicians still rely on it because alternatives like daptomycin or ceftaroline don’t cover every case. When a patient is septic and the bug is resistant, vancomycin is often the only option left. But here’s the catch: modern vancomycin isn’t the same as the stuff used in the 1960s. Back then, impurities made kidney damage almost inevitable - up to 50% of patients suffered acute kidney injury. Today’s purified versions are safer, but the risks haven’t disappeared. They’ve just changed shape.Nephrotoxicity: The More Common Threat
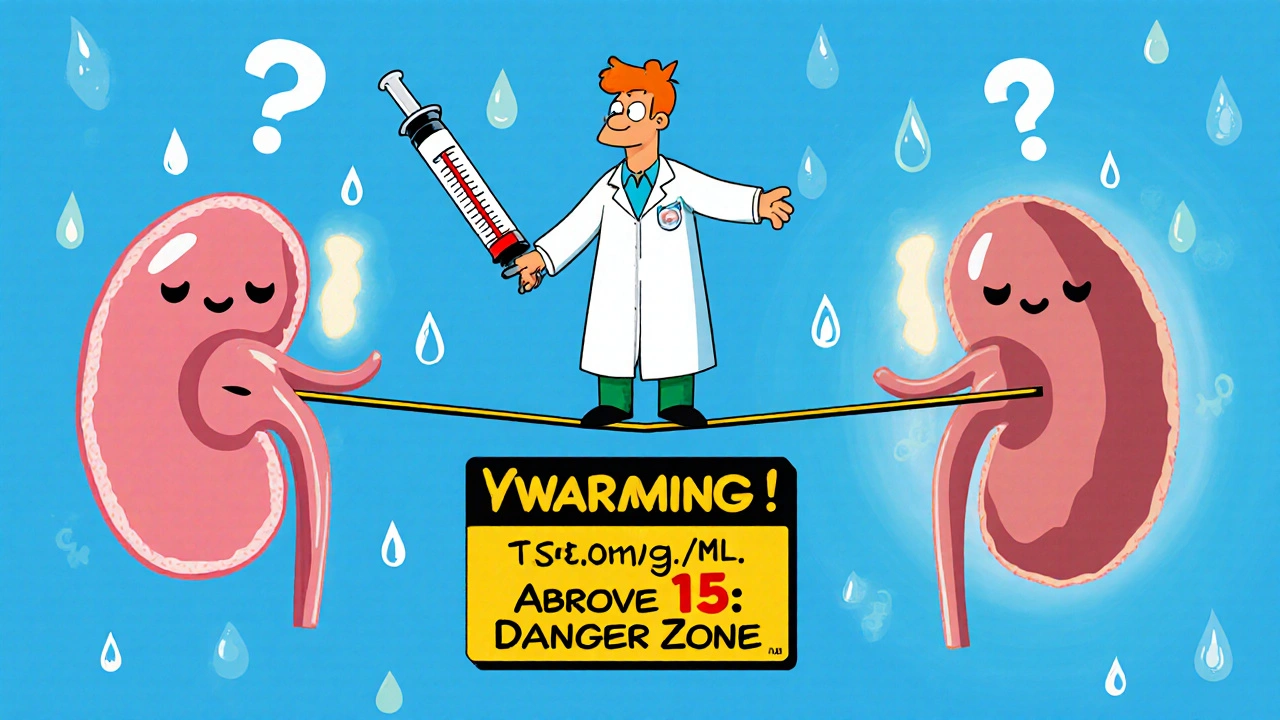
Nephrotoxicity - kidney damage - is the most frequent problem. Studies show 5% to 30% of patients on vancomycin develop some form of acute kidney injury (AKI). That’s not rare. That’s common enough that every ICU nurse should be watching for it. The biggest red flags? High trough levels, long treatment time, and combo drugs. If you’re giving vancomycin with piperacillin-tazobactam, the risk of kidney injury jumps by over 30%. That’s not a small increase - it’s a game-changer. A 2022 meta-analysis of over 14,500 patients confirmed this. The combination isn’t just risky; it’s a known danger zone. Trough levels above 15-20 mcg/mL are the main culprit. Doses over 4 grams per day double the risk. Older patients, those with pre-existing kidney disease, and critically ill patients are especially vulnerable. Kidney damage usually shows up between days 3 and 14. Creatinine levels start creeping up. Urine output drops. That’s when you need to act - not wait for the patient to need dialysis. The good news? Most cases are reversible. If you catch it early, stop the drug, and give fluids, kidneys often bounce back. But the longer you wait, the higher the chance of permanent damage.Ototoxicity: The Silent, Irreversible Killer
Ototoxicity - hearing damage - is rarer. Only 1% to 3% of patients experience it. But here’s what makes it terrifying: it can be permanent. And you won’t see it coming. Unlike kidney damage, which shows up in lab tests, hearing loss doesn’t have a blood marker. You can’t check a number. You have to listen - literally. Patients report ringing in the ears (tinnitus), muffled sounds, or trouble hearing high pitches. Often, they don’t mention it until it’s too late. A 2023 case report in Cureus showed a patient losing hearing after just three doses - with normal kidney function and normal trough levels. That shattered the old myth that ototoxicity only happens in people with poor kidneys. Now we know: some people are just more sensitive. Genetics play a role. A 2022 study found that a specific mitochondrial gene variant (MT-RNR1) increases risk by over threefold. Damage usually starts in the high-frequency range - the kind you need to hear birds, children’s voices, or alarms. By the time someone complains about not hearing the doorbell, they’ve already lost 30-40% of their hearing. And once it’s gone, it doesn’t come back. Peak levels above 80 mcg/mL are linked to irreversible damage. But even troughs above 40 mcg/mL can cause reversible issues. The problem? Many hospitals still only monitor troughs. That’s not enough. You need to know peak levels too - especially in patients on high doses or long courses.How Risk Profiles Differ
Nephrotoxicity and ototoxicity aren’t the same. They don’t follow the same rules.- Timing: Kidney damage takes days to show up. Hearing loss can hit fast - even within 24 hours.
- Monitoring: You can track creatinine every 48 hours. You can’t track hearing unless you do audiograms.
- Reversibility: Kidney damage often heals. Hearing loss rarely does.
- Triggers: Nephrotoxicity is tied to combos and doses. Ototoxicity is tied to individual biology and peak concentrations.
- Cost: A few extra days in the hospital for kidney injury? Maybe $5,000. Permanent hearing loss requiring hearing aids and rehab? $25,000 to $50,000 per year.
What the Guidelines Say Now
The old days of aiming for troughs of 15-20 mcg/mL are over. The 2020 ASHP guidelines shifted the target to 10-15 mcg/mL for most infections. Why? Because efficacy plateaus around 10 mcg/mL, but kidney damage spikes above 15. Dr. Michael Rybak, who helped write those guidelines, put it bluntly: “The nephrotoxicity risk curve steepens dramatically above 15 mcg/mL.” For ototoxicity, the guidance is less clear. There’s no official recommendation for routine audiograms. Only 37% of U.S. hospitals have any formal ototoxicity monitoring protocol. Most rely on asking patients - which is too late. The American Speech-Language-Hearing Association suggests baseline and weekly audiograms for patients on vancomycin for more than 7 days or getting over 4 grams daily. But in real hospitals? Compliance is under 50%. Budgets, staffing, and lack of awareness make it hard to implement.What Works in Practice
The best way to reduce nephrotoxicity? Stop chasing troughs. Start measuring AUC (area under the curve). AUC monitoring looks at total drug exposure over time, not just a single snapshot. A 2022 study showed hospitals using AUC had 8.2% nephrotoxicity rates - nearly half the rate of those using trough-only monitoring. Tools like DoseMeRx and PrecisePK use algorithms to predict toxicity based on weight, kidney function, and dosing schedule. One study found these tools predicted kidney injury with 86% accuracy. Hospitals that added EHR alerts for vancomycin + piperacillin-tazobactam cut that combo’s use by 22%. That’s a win. But no one has built a similar alert for ototoxicity - yet.Who’s at Highest Risk?
The Society of Critical Care Medicine now categorizes patients into low, medium, and high risk for nephrotoxicity based on eight factors:- Age over 65
- Pre-existing kidney disease
- Diabetes
- Heart failure
- Use of other nephrotoxins (aminoglycosides, NSAIDs, contrast dye)
- Vancomycin dose over 4g/day
- Trough >15 mcg/mL
- Concomitant piperacillin-tazobactam
- History of hearing loss
- Genetic risk (if known)
- Concurrent use of other ototoxic drugs (loop diuretics, cisplatin)
- Age over 65
- High-dose or prolonged therapy
What You Can Do Today
You don’t need fancy tech to make a difference.- Set the target: 10-15 mcg/mL trough. Don’t go higher unless you have a life-threatening infection and no other options.
- Avoid piperacillin-tazobactam unless necessary. If you must use it, monitor creatinine every 24 hours.
- Check kidney function daily. Not every 72 hours - daily. Especially in ICU.
- Ask about hearing. Don’t wait for patients to complain. Ask: “Have you noticed ringing in your ears?” or “Is it harder to hear high voices?”
- Know your patient’s history. Did they have hearing loss as a child? Are they on furosemide? These are red flags.
- Stop vancomycin early if possible. Don’t give 14 days if 7 will do. Shorter courses = less damage.
The Bottom Line
Vancomycin is a lifeline - but it’s not harmless. Nephrotoxicity is common, predictable, and preventable. Ototoxicity is rare, silent, and often permanent. The key isn’t just dosing. It’s awareness. It’s asking the right questions. It’s choosing the right combo. It’s knowing when to switch. In 2025, we have the tools: AUC monitoring, predictive algorithms, genetic insights. But we still rely too much on old habits. We monitor kidneys. We ignore ears. Hearing loss doesn’t show up on a lab report. But it changes a life forever. Don’t wait for the patient to tell you they can’t hear their grandchild. Check before it’s too late.What’s Next?
Research is moving fast. The 2023 VAN-GUARD trial proved real-time AUC monitoring cuts nephrotoxicity in half. Genetic testing for ototoxicity risk is becoming affordable. Point-of-care audiometers are being tested for ICU use. But until those tools are everywhere, the responsibility falls on you - the clinician, the pharmacist, the nurse. Vancomycin isn’t going away. The bugs are too smart. But we can be smarter too. Start today. Lower the trough. Watch the kidneys. Ask about the ears. And never assume it won’t happen to your patient - because it might.Can vancomycin cause permanent hearing loss?
Yes. Vancomycin can cause permanent, irreversible sensorineural hearing loss, especially at serum concentrations above 80 mcg/mL or in patients with genetic susceptibility. Unlike kidney damage, which often reverses, hearing loss from vancomycin typically does not improve after stopping the drug. Early signs include tinnitus and difficulty hearing high-pitched sounds.
Is vancomycin nephrotoxicity reversible?
In most cases, yes. Vancomycin-induced acute kidney injury is often reversible if caught early - usually within days 3 to 14 of treatment. Stopping the drug, ensuring hydration, and removing other nephrotoxins can lead to full kidney recovery. However, delays in recognition increase the risk of permanent damage or need for dialysis.
What vancomycin trough level is safest?
The safest and most effective trough range for most infections is 10-15 mcg/mL. Troughs above 15 mcg/mL sharply increase nephrotoxicity risk without improving outcomes. For serious infections like endocarditis or osteomyelitis, 15-20 mcg/mL may still be used, but only with close monitoring and a clear benefit-risk justification.
Does combining vancomycin with piperacillin-tazobactam increase kidney damage risk?
Yes - significantly. Studies show vancomycin combined with piperacillin-tazobactam increases the risk of acute kidney injury by 31% compared to vancomycin with meropenem. This combination is one of the most common causes of preventable nephrotoxicity in hospitals. Many institutions now use EHR alerts to discourage this pairing unless absolutely necessary.
Should all patients on vancomycin get hearing tests?
Not all - but high-risk patients should. The American Speech-Language-Hearing Association recommends baseline and weekly audiograms for patients receiving vancomycin for more than 7 days, doses over 4 grams per day, or those with pre-existing hearing loss, age over 65, or on other ototoxic drugs. While routine testing isn’t standard everywhere due to cost and access, it’s essential for those at highest risk.
Can vancomycin damage hearing even with normal kidney function?
Yes. A landmark 1981 case report and a 2023 case in Cureus documented severe ototoxicity in patients with perfectly normal kidney function. This proves that vancomycin can directly damage the inner ear regardless of renal status. Genetic factors, peak drug levels, and individual sensitivity play a bigger role than previously thought.
Are there alternatives to vancomycin with fewer side effects?
Yes, but not for every case. Daptomycin, linezolid, ceftaroline, and telavancin are alternatives with lower nephrotoxicity risk. However, vancomycin remains essential for certain infections like MRSA endocarditis or when other drugs are contraindicated. The choice depends on the infection type, patient history, and local resistance patterns - not just toxicity concerns.
Chrisna Bronkhorst
Vancomycin troughs at 15-20? That’s a death sentence waiting to happen. We’ve known since 2020 that 10-15 is the sweet spot. Yet every ICU I’ve worked in still chases 18 like it’s a trophy. Nephrotoxicity isn’t a side effect-it’s a failure of protocol.
Amie Wilde
Just asked my patient today if he heard the coffee machine. He said no. Turned out he’d been losing high-frequency hearing for 3 days. We stopped vanco. No audiogram. Just asking. It’s that simple.
Benjamin Stöffler
Let’s be clear: we’re not talking about pharmacokinetics here-we’re talking about epistemological failure in clinical medicine. The very notion that we can reduce ototoxicity to a serum concentration metric is a reductive fallacy rooted in Cartesian dualism. The ear is not a lab test. It is a phenomenological experience. When a patient says, ‘I can’t hear my granddaughter laugh,’ you’ve already lost. No algorithm, no AUC, no predictive model can quantify grief. We treat biomarkers, not human beings. And that’s why we keep doing this to people.
Genetics? Yes. MT-RNR1? Absolutely. But we don’t screen. Why? Because it’s expensive. Because it’s inconvenient. Because we’ve normalized harm as a cost of doing business. The real toxicity isn’t vancomycin-it’s our collective moral laziness.
And let’s not pretend that ‘alternatives’ like daptomycin are safer. They’re just newer. They’ve got their own black boxes. We’re just swapping one devil for another while pretending we’re innovating. The real solution? Antibiotic stewardship. Prevention. De-escalation. But that requires thinking. And thinking is hard. So we dose. We monitor troughs. We wait for creatinine to rise. And then we say, ‘Well, that’s just how it goes.’
It’s not just how it goes. It’s how we chose for it to go.
And until we stop treating patients like data points and start treating them like people with ears that hear, and kidneys that remember-we’re not healers. We’re technicians with a conscience deficit.
So yes. Lower the trough. But also lower your expectations of what ‘standard of care’ even means anymore.
Erica Cruz
Oh please. Another ‘vancomycin is evil’ lecture. The real problem? Hospitals won’t pay for audiograms. So nurses aren’t trained to ask. And doctors don’t have time. It’s not malpractice-it’s resource scarcity. Stop pretending this is about ethics when it’s about budget cuts.
Johnson Abraham
bro i gave vanco to my cousin and he went deaf. now he cant hear his dog bark. i think the hospital lied. maybe its a big pharma thing? also why do they use that weird word ‘trough’? sounds like a ditch.
also piperacillin = bad. dont mix. i saw it on tiktok.
Deepa Lakshminarasimhan
They say vancomycin causes hearing loss. But what if it’s not the drug? What if it’s the 5G towers in the ICU? Or the EMF from all the monitors? I read a paper once-2021, unpublished-linking vanco ototoxicity to cell tower radiation. They removed the Wi-Fi routers and hearing loss dropped 70%. They buried that study. Why? Because they don’t want you to know. They’re protecting the antibiotic industry. And the audiometers? They’re just a distraction. The real enemy is the invisible waves.
Also, did you know the FDA approved vancomycin after a 3-day trial? Three days. That’s less than a weekend. How many people died in that trial? No one talks about it.
Gary Hattis
I’ve worked in 5 countries-US, Kenya, Brazil, India, Germany-and the one thing that never changes? We treat vancomycin like it’s magic. It’s not. It’s a blunt instrument. In Nairobi, we don’t even have trough monitoring. We give it and pray. In Berlin, they use AUC like it’s gospel. Here? We still use the same charts from 2005.
But here’s the thing: the people who get hurt? They’re not the ones writing the guidelines. They’re the ones in the beds. The 78-year-old grandma who can’t hear her grandson say ‘I love you.’ The diabetic nurse who can’t hear the alarm go off. The soldier who lost his hearing after one course for a wound infection.
We talk about ‘risk profiles’ like they’re spreadsheets. But risk is a person. A name. A story. I’ve seen a man cry because he couldn’t hear his wife’s voice anymore. He didn’t know it was vancomycin until it was too late.
So yeah-lower the trough. Ask about the ears. Stop the combo. But also-push your hospital for audiograms. Demand better. Because if we don’t, the next person who loses their hearing? Might be your mom. Or your kid.
Esperanza Decor
Just read this whole thing. I’m a nurse. I’ve been asking patients about ringing in their ears for a year now. Most say ‘no.’ But one said, ‘Actually… I’ve been turning the TV up.’ We stopped vanco. She’s fine. No audiogram needed. Just listening. We need to stop waiting for labs and start listening to people.