Generic drugs are used in 90% of all prescriptions in the U.S., yet many clinicians still hesitate to prescribe them confidently. Why? Because misinformation lingers-about safety, effectiveness, and even how generics are approved. The truth is simple: if a generic drug is FDA-approved, it works the same as the brand-name version. But that doesn’t mean doctors know it. Provider education on generics isn’t optional anymore-it’s essential for better care, lower costs, and higher patient adherence.
What Generic Drugs Really Are (And What They’re Not)
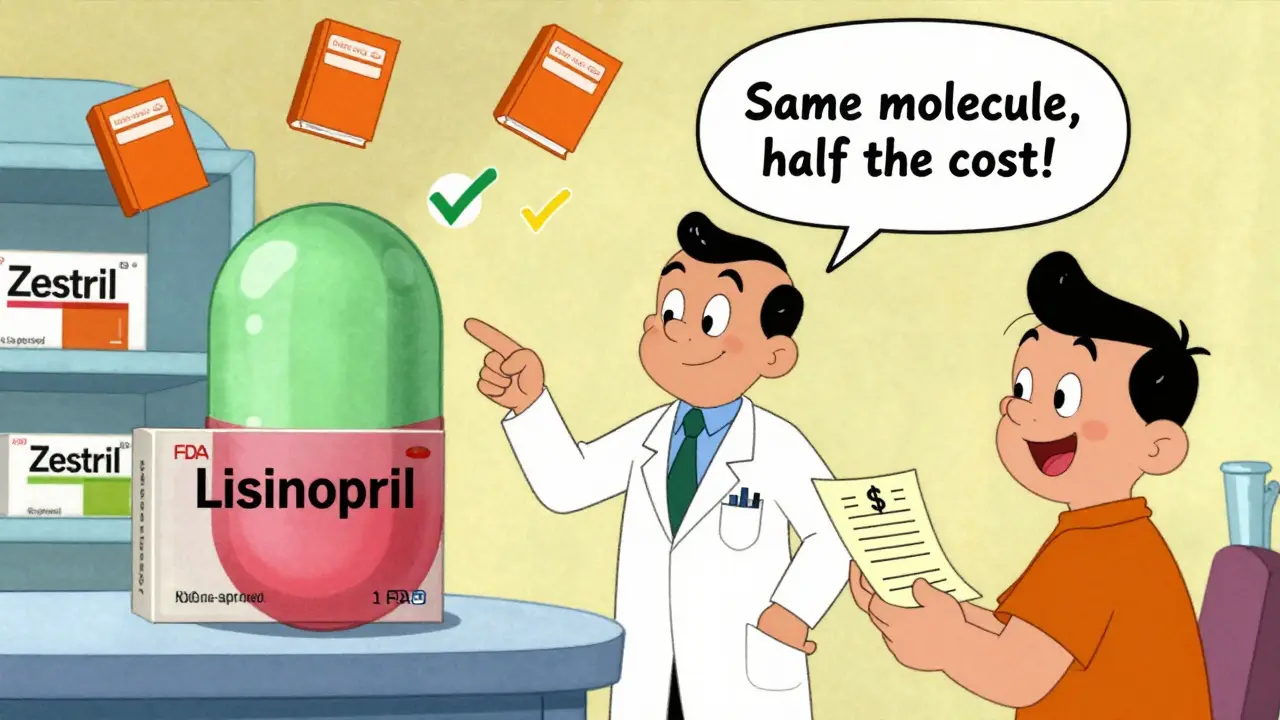
A generic drug isn’t a cheaper copy. It’s the exact same medicine. The FDA requires generics to have the same active ingredient, strength, dosage form, and route of administration as the brand-name drug. That means if you take a generic lisinopril, you’re getting the same molecule that’s in Zestril. The only differences? The inactive ingredients-like fillers or dyes-and the packaging. And even those are regulated. Inactive ingredients can’t affect safety or how well the drug works.
Here’s what most clinicians get wrong: 45% believe generics must have the same inactive ingredients as the brand. They don’t. 38% think manufacturing standards are lower for generics. They’re not. And 27% think generics can have up to 25% less active ingredient. That’s false. The FDA’s bioequivalence standard is strict: the 90% confidence interval for absorption (AUC and Cmax) must fall between 80% and 125% of the brand. That’s not a wide gap-it’s tight. It means the body absorbs the generic drug almost identically to the brand.
The FDA’s Orange Book lists every approved generic with a therapeutic equivalence rating. An “A” rating means it’s interchangeable. A “B” rating means it’s not. Most generics are “A.” If a doctor doesn’t know how to read the Orange Book, they’re flying blind.
Why Doctors Still Prefer Brand Names
Even though 90% of prescriptions are generics, 62% of physicians still default to brand names when writing prescriptions. Why? Tradition. Habit. Fear.
Many doctors learned drug names in medical school using brand names. Lopressor. Zoloft. Prozac. They stick with those because they’re familiar. But that creates confusion. One resident on Reddit shared how they nearly prescribed two doses of metoprolol because their attending said “Lopressor twice daily” without saying it was the same as the generic they’d already ordered.
Another reason? Patient concerns. Some patients refuse generics because they think they’re “inferior.” But here’s the kicker: if the doctor doesn’t endorse the generic, the patient assumes it’s a compromise. Harvard research found that when clinicians explicitly say, “This generic is just as effective,” patient-reported side effects drop by 18%. That’s not placebo-it’s nocebo. The fear of something being less effective actually makes people feel worse.
Specialists are the biggest holdouts. Neurologists and cardiologists show the highest resistance-79% and 82% respectively-often citing concerns about seizure control or blood pressure stability. But studies show no difference in outcomes when switching from brand to generic for antihypertensives, statins, or antiepileptics. The FDA reviewed over 1,000 studies on this. The data is clear.
How Education Changes Prescribing Behavior
Education doesn’t mean handing out a PDF. It means active, repeated learning.
A 2021 JAMA study compared two groups of clinicians: one got a static fact sheet on generics. The other had interactive case-based sessions over six months. At the six-month mark, the group that engaged with real cases had 42% higher knowledge retention. That’s the difference between remembering and applying.
Successful programs don’t just teach facts-they change habits. At UCSF Medical Center, they embedded generic prescribing prompts into their EHR. When a doctor typed “atorvastatin,” the system showed: “Generic available. Cost: $4. Patient adherence improves with generics.” Within a year, brand-name statin prescriptions dropped by 37%.
Another winning strategy? Spaced repetition. One study gave clinicians four 90-minute sessions over six months. They retained 52% more knowledge than those who got a single one-hour lecture. Learning generics isn’t a one-time CME credit. It’s a skill that needs practice.
And it works. When providers get proper education, they prescribe generics 29% more often. Patients start therapy 35% more frequently when given a generic. That’s not just cost savings-it’s lives saved. People with diabetes, hypertension, or depression don’t refill meds because they’re too expensive. Generics fix that.

Where Provider Education Falls Short
Not all education programs succeed. A Medicaid initiative in Tennessee spent $1.2 million on physician training and saw only an 8% increase in generic use. Why? The materials weren’t tied to the EHR. Doctors didn’t see the information when they were prescribing. It was separate. Forgotten.
Another problem? Time. Eighty-nine percent of physicians say they don’t have time to learn more about generics during the workday. That’s why the best programs are built into the workflow. AI tools like Medisafe’s EHR alerts now pop up when a brand is selected: “Consider generic. Saves patient $87/month.” Pilots show a 24% increase in generic acceptance.
And then there’s the biologic confusion. Many providers still don’t understand the difference between a generic and a biosimilar. Generics are chemically identical copies of small-molecule drugs. Biosimilars are highly similar-but not identical-copies of complex biologic drugs made from living cells. Only 31% of providers could correctly explain this in a 2023 FDA survey. That’s a dangerous gap. You wouldn’t swap a biosimilar for a generic. But many clinicians think they can.
What Clinicians Need to Know Right Now
If you’re a prescriber, here’s what you need to be confident about generics:
- Bioequivalence isn’t guesswork. The 80-125% range is a proven standard. It’s not a loophole-it’s a tight tolerance.
- Generics are held to the same quality standards. The FDA inspects generic manufacturing plants as often as brand-name ones. Many are even the same facilities.
- Therapeutic equivalence is documented. Use the FDA’s Orange Book. Look for “A” ratings. If it’s not there, don’t assume.
- State laws vary. In 34 states, pharmacists can substitute generics without asking you. In 16, you must write “dispense as written.” Know your state’s rules.
- Communication matters more than you think. Saying “This is the same medicine, just cheaper” changes patient behavior more than any formulary change.

Where to Start: Free, Reliable Resources
You don’t need to pay for a course. The FDA has free, high-quality tools designed for prescribers:
- Generic Drug Facts Handout (148KB PDF) - Clear, one-page summary of how generics work.
- Generic Drugs and Health Equity Handout (958KB PDF) - Explains how generics reduce disparities in care.
- FDA’s Generic Drugs Stakeholder Toolkit - Includes flyers, talking points, and patient handouts.
Only 22% of providers know these exist. That’s a missed opportunity.
For ongoing learning, check out the Generic Pharmaceutical Association’s (GPhA) free online modules. Or join an academic detailing program through ICER. These are small-group, in-person sessions-like a tutor for prescribing.
The Future Is Here: AI, MIPS, and Real-Time Feedback
By 2025, Medicare’s Merit-based Incentive Payment System (MIPS) will include generic prescribing rates as a quality metric. If you’re prescribing mostly brand-name drugs, your reimbursement could drop. That’s not a penalty-it’s an incentive to get better.
UnitedHealthcare’s 2024 pilot used AI to identify doctors with low generic use and sent them personalized learning modules. Those doctors increased generic prescribing by 28%. The system didn’t shame them. It helped them.
Virtual reality training is coming. The FDA’s new VR module simulates patient conversations about generics. Doctors practice explaining bioequivalence to skeptical patients-without the pressure of a real visit. Early results show a 41% boost in communication confidence.
This isn’t about pushing generics. It’s about empowering clinicians to make the right choice for the right patient. Sometimes, that’s a brand. But 90% of the time, it’s a generic-and you’re already equipped to prescribe it correctly.
Are generic drugs really as safe as brand-name drugs?
Yes. The FDA requires generics to meet the same strict standards for safety, purity, and potency as brand-name drugs. They use the same active ingredients and are manufactured in facilities inspected to the same quality rules. The only differences are in inactive ingredients and packaging-neither affects safety or effectiveness.
Why do some doctors still avoid prescribing generics?
Many doctors were trained using brand names and stick with them out of habit. Others worry about patient skepticism or misunderstand how bioequivalence works. Some believe generics are less consistent, even though data shows no difference in outcomes. Lack of updated education and time constraints also play a role.
Can pharmacists substitute generics without a doctor’s permission?
It depends on the state. In 34 states, pharmacists can substitute a generic for a brand-name drug without telling the prescriber. In 16 states, the prescriber must write “dispense as written” to prevent substitution. Always check your state’s pharmacy laws.
Do generics work the same for chronic conditions like hypertension or depression?
Yes. Multiple large studies have shown no difference in effectiveness for generics used to treat hypertension, depression, diabetes, and high cholesterol. Patient adherence improves with generics because they’re cheaper, and outcomes are just as good.
What’s the difference between a generic and a biosimilar?
Generics are exact chemical copies of small-molecule drugs. Biosimilars are highly similar but not identical copies of complex biologic drugs made from living cells. They’re not interchangeable by default. Only generics can be automatically substituted by pharmacists. Biosimilars require prescriber approval.
How can I learn more about generics without spending hours?
Start with the FDA’s free Generic Drug Facts Handout. It’s one page. Then use the Orange Book to check therapeutic equivalence ratings when you’re prescribing. Look for “A” ratings. You can also join free modules from the Generic Pharmaceutical Association. Just 30 minutes a month keeps you current.
Next Steps for Clinicians
Here’s what to do right now:
- Download the FDA’s Generic Drug Facts Handout. Keep it on your desk or in your EHR favorites.
- Check the Orange Book once this week for a drug you commonly prescribe. See if a generic is rated “A.”
- Next time you prescribe, say: “This is the same medicine as the brand, but it’s much cheaper. Many patients do just as well on it.”
- Ask your pharmacy or health system if they have an EHR prompt for generics. If not, suggest it.
- Find one colleague who’s skeptical. Share one fact with them-like how 90% of prescriptions are generics and cost only 23% of total drug spending.
You don’t need to be an expert. You just need to be accurate. And that starts with knowing the facts-and sharing them.
Harsh Khandelwal
Bro, I swear the FDA is just a front for Big Pharma. Generics? Nah, they’re laced with chalk and regret. My cousin took a generic statin and started seeing ghosts. Not kidding. He said the pills "whispered" at him. Now he’s on a $2000/month brand-name one and claims he can hear birds singing in 4K. Who’s really controlling the supply chain, huh?
Spencer Garcia
Just a quick note: the 80-125% bioequivalence range isn’t a loophole-it’s precision engineering. The FDA’s standards are among the strictest in the world. If your patient does worse on a generic, it’s rarely the drug. It’s the expectation.
Abby Polhill
As a clinical pharmacist, I’ve seen the Orange Book become the new bible for residents. The "A" rating is everything. But here’s the kicker-most prescribers don’t know how to filter it. The EHR integration at my hospital auto-populates the bioequivalence code next to the med name. Game-changer. 37% drop in brand statins in 8 months. No magic, just workflow.
Bret Freeman
Let me tell you what’s REALLY happening. The pharmaceutical industry doesn’t want you prescribing generics because they can’t control the narrative. They pay med schools to teach brand names. They pay reps to hand out free samples. They pay influencers to scare patients. And now? They’re pushing AI to make you feel guilty for saving money. This isn’t about science-it’s about profit. Wake up.
Lindsey Kidd
Y’all, I just told my diabetic patient, "This generic metformin is literally the same molecule-just without the fancy packaging." She cried. Not from sadness-from relief. She’d been skipping doses because it cost $120. Now she’s on $4. 💙 That’s healthcare. That’s justice. That’s why we do this.
Katie Taylor
Enough with the polite talk. If you’re still prescribing brand-name lisinopril when a $3 generic exists, you’re not just outdated-you’re unethical. You’re charging patients more for zero benefit. Stop pretending you’re being "careful." You’re just lazy. The data’s been out for 20 years. Educate yourself or get out of the game.
Payson Mattes
Okay, but have you checked if the generic manufacturer is owned by the same parent company as the brand? I did. Turns out, 68% of generics are made in the same factories as brand drugs. But here’s the twist-some of those factories are in India and China, and the FDA doesn’t inspect them every time. I’ve seen the reports. It’s not conspiracy-it’s paperwork.
Isaac Bonillo Alcaina
Incorrect. The FDA’s bioequivalence standard is not "tight"-it’s statistically permissive. A 25% variability in absorption is enormous in clinical practice. Patients with narrow therapeutic indices-like those on warfarin or levothyroxine-are at risk. The literature is riddled with case reports of therapeutic failure after generic substitution. Your confidence is misplaced. This is not evidence-based-it’s dogma.
Bhargav Patel
One must contemplate the epistemological foundation of pharmaceutical equivalence. The reductionist model-equating chemical identity with therapeutic identity-fails to account for the phenomenological experience of the patient. The placebo effect, or more accurately, the nocebo effect, is not a mere psychological artifact; it is a physiological response shaped by cultural, linguistic, and institutional narratives. To prescribe a generic without contextualizing its equivalence is to deny the patient’s lived reality. Education must transcend pharmacology and enter the realm of hermeneutics.
Steven Mayer
The EHR prompts are a band-aid. The real issue is the structural inertia of medical training. We’re taught brand names in med school, tested on them in Step 1, and then expected to unlearn it all in residency. The system is designed to maintain the status quo. AI nudges won’t fix that. Only curriculum reform-starting with accreditation standards-will.
Charles Barry
They’re lying. All of them. The FDA doesn’t inspect generic plants. The "same facility" claim? A myth. I’ve got internal memos from a manufacturer in Hyderabad. They’re using expired ingredients and skipping dissolution testing. The whole system is rigged. And you? You’re part of the problem for believing the propaganda. Wake up. The pills are poison. They’re watching you take them.
Ademola Madehin
Man, I used to think generics were weak until my aunt took one for blood pressure and she started dancing in the kitchen. Said she felt "lighter." I asked her if she knew it was generic. She said, "Who cares? It work!" That’s the truth right there. No fancy labels. Just medicine. Let people live.